Authorised Representation / FBO/ RP Process Flow
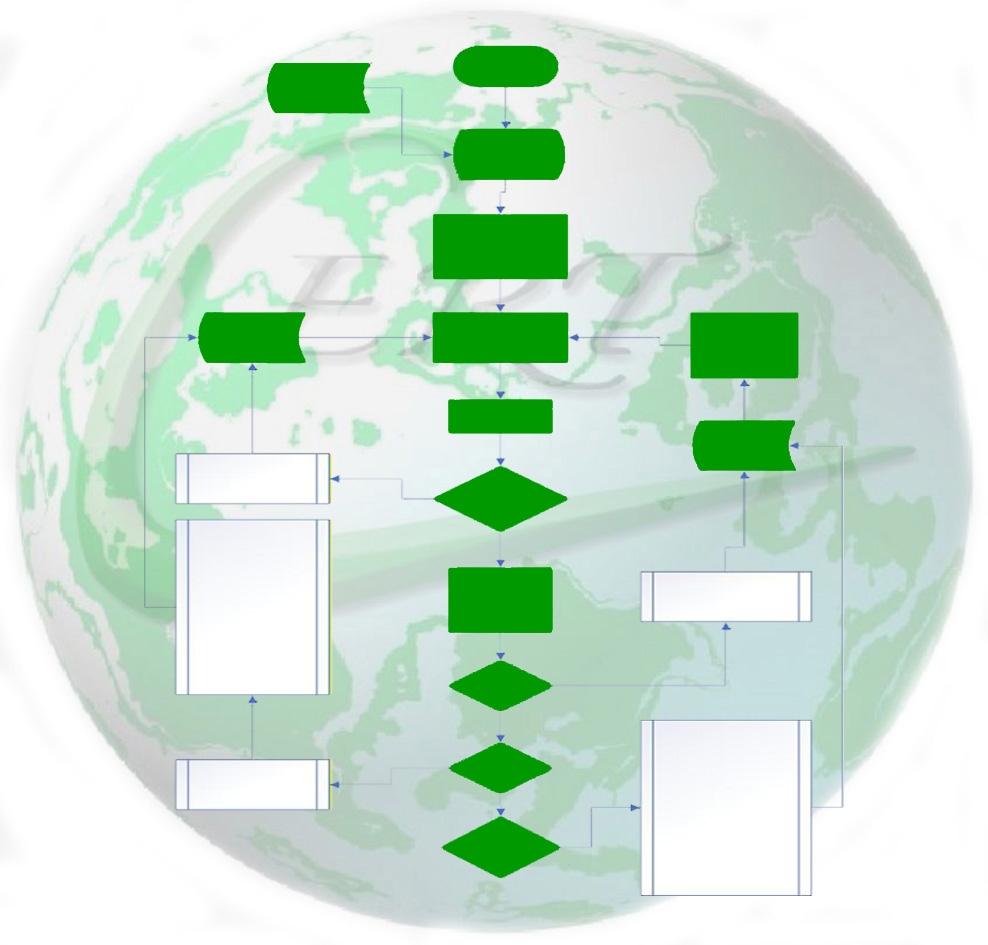
Product Information Folder (PIF) created using the template provided in your secure filing in the Product Files folder.
Usually one folder per SKU being represented.
External data can be registration certificates, declarations of conformity or test reports needed to substantiate the product safety and suitability for intended markets.
Product Information Files (PIF) created using template.
Product Labelling & Documentation updated with CERT address and any other mandatory markings needed.
CERT Regulatory Adviser to be invited via the Product Schedule to review the Product Information File (PIF).
To do this: change 'Review my Project' to "Yes" and click on 'send email' to notify the relevant team.
CERT Regulatory Team to review the product information to confirm compliance with the markets you intend to sell in.
Product reviewed based on markets indicated on the Product Schedule and either released to be sold on the market or additional changes requested.
PIF updated with confirmation in writing (signed and dated report) confirming the markets the product has been assessed and approved to be sold in.
Products cannot be released to the market without this!
If you need to add New Markets?
This is common practice and encourage this as it is a sign of growth. We like to see our clients prosper.
Stop the representation service? You can stop the service for a single SKU or the entire service with 30-days' notice or when the product is no longer on sale on the market - whichever is the latter.
If you have any alterations to the product, you need to notify us of the change. This can easily be done by updating the filing with the change, mark the 'Ready to Review' as Yes and notify us via the Product Schedule.
Additional information must be added to the PIF to support the product changes.
Old information can be replaced with new documentation. You are responsible for maintaining a paper trail for changes to products over time.
You can create an archive folder to add redundant information into, but redundant information must not be present where it can be mistaken as current.
Additional Markets need to be added to the Product Schedule so we have visibility of the additional markets needing confirmation and approval.
The additional data should be added to the Product Information File (PIF). Please ensure all information present is current and relevant.
Old data can be stored in a sub-folder called 'Archive' if relevant for traceability. The current information will be shared with Market Authorities on request.
Please change the 'Product Status' in the Product Schedule to "Yes" and click on 'send email' to notify us the product is ready to be reviewed.
30-days' notice on email needs to be provided to your nominated Regulatory Adviser to stop the service (as per the Service Ag reement) to cater for the administration.
This can be for an individual SKU or for the whole service.
If you temporarily stopped the service and want to start again, please let your CERT Regulatory Adviser know as soon as possible.
Products must not be released on the market without approval or representation service started.
If there are updates to the product these will need to be reviewed and approved to ensure legal support.
Updates - Please change the ‘Ready to Review’ column to “Yes” and click on ‘send email’
No changes – Add a comment date and request in comments section to start the service and confirm the date the service is to start.
Changes are provided in a documented change request.
This is normally emailed directly to the project submitter with mandatory change requirements clearly identified.
The updated data needs to be added to the Product Folder in the correct sub-folder sections.
Please ensure old data is filed in an Archive Folder or removed, so only curreny information is visible.
