

The process in 4 easy steps:
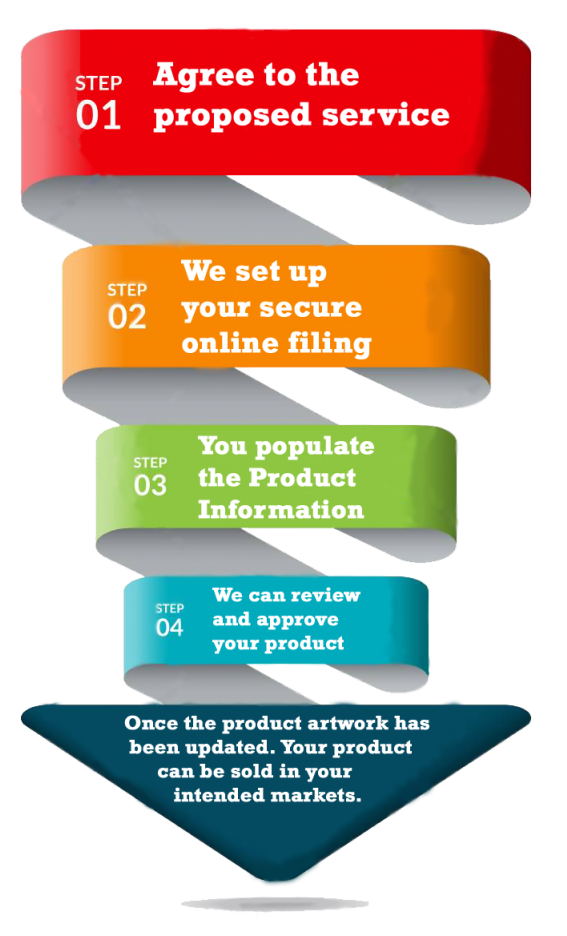
CERT is not the consignee which means we are not acting as the full importer. We provide product legal market representation with the objective to provide you with as much flexibility as possible, by allowing you full control of who you wish to sell to, your shipping, warehousing and distribution.
This is deliberate as each customer has negotiated their own shipping and distribution choices, which we do not want to interfere with. You maintain full ownership of your product and can choose who acts as the Importer of Record to deal with the customs and excise and addressing your Extended Producer Responsibilities (EPR). This could be your shipping company, warehousing, or your client.
We believe this approach gives you full control over your product and free choice within the EU or UK market to sell to whoever you wish.
CERT aims for a clear simple approach to your regulatory requirements, allowing you to focus on your core business. A one price fits all would have been great, but not practical; as products vary in complexity and this would have penalised a lot of product areas having to subsidise more complex products.
So, the most cost-effective and simple approach to it is to split the charge into two stages:
- Initial review
- Monthly service charge.
This allows us to offer you a simple fixed charge for the Authorisation Service which matches the way we work.
How do the two stages work?
The review charge is a fixed one-off charge defined by the complexity of your product and the number of markets required:
| Complexity Classification | Base Definitions | Examples |
SimpleSimple product testing requirements with minimal/generic regulatory requirements e.g. General Product Safety Regulations, Weights and Measures Act. | Food: Up to 10 ingredients. No processing other than cutting, chopping, bottling or squeezing. No nutritional claims. | Food Examples: Fresh produce, milk, cheese, fish, chopped vegetables, raw meat, orange juice. |
| Supplements: Up to 3 of the core approved vitamins and minerals ingredients selling in any of the EU countries & UK. | Supplement Examples: Magnesium Citrate supplement. | |
| Non-food: Single ingredient composition/article or very simple multi-component. | Non-food Examples: Paper cups/plates, crockery, cutlery, food packaging, bags, simple component care and beauty (cotton pads, hair bands, nail files, tweezers), simple textiles (towels, bedding, cloths), simple component hygiene products). | |
| —————- | —————- | —————- |
Medium | Food: Added value products that are processed. All in one mixing bowl stage, with or without simple Protein. | Food Examples: Yoghurt, mousses, soups, pates, fish with sauce, cooked meats, bread, mashed potatoes. Sausages, burgers. Food powders, plain chocolate bars. Food supplements. |
| Supplements: 3-8 nutritionally active ingredients, which include up to 3 herbal/plant extracts and selling in one of the core EU markets* & the UK. | Supplement Examples: ADEK complex with Lions Mane, Bilboa, Turmeric, Essential Amino Acids blend. | |
| Non-food: More complex warning labelling. Products consisting of basic mixtures with potential low level/no irritants. | Non-food Examples: CLP exempt mixtures, simple toy labelling requirements, medium complex hygiene products (razors, tampons, nappies, toothbrushes, dental accessories, bandages), baby accessories, upholstery. | |
| —————- | —————- | —————- |
Complex | Food: Added value products with more than one recipe, component or cooking method. | Food Examples: Ready meals, pies, cakes, multipacks of biscuits/confectionery, sandwiches. |
| Supplements: Over 8 nutritionally active ingredients, which includes over 3 herbal/plant extracts selling in any of the EU countries. | Supplement Examples: Multivitamins, Probiotic blends, Plant complex based food supplements. | |
| Non-food: High risk products. Mixtures with registered sensitisers that require CLP statements/warnings. Biocides. Multi-component products. Domestic electrical and battery operated products. | Non-food Examples: Make-up, skin care, hairspray, shave gel, wipes, toothpaste, mouthwash, cleaning and detergents, first aid, monitors, pregnancy test kits, pet food, fragrance products, complex toy labelling, gift sets/multi-component sets, small domestic appliances, white goods, infra-red, bluetooth, wi-fi devices. |
Factors that affect the cost:
Additions to the review time will have a corresponding increase in cost.
- Products that have harmonised standards in different markets, will reduce the review time.
- The quantity of markets the product needs to comply with will affect the review time.
- As an example – asking for all of EU will mean a cross-check for compliance against 27 countries which will increase review time. We recommend requesting for your immediate/short-term markets as you can always add extra markets at a later stage as a minor update to the project.
- Products that have ‘cross-over‘ i.e. only have minor changes e.g. colour/quantity/size that do not affect the formulation/ingredients or regulatory requirements, are subject to discount when submitted at the same time for review:
- 1st Submission – 100%
- Product 2 (cross-over) – 50% of the review cost
- Product 3 onwards (cross-over) – 25% of the review cost
- Products submitted at different time intervals are not subject to cross-over discount, but a discount may be applied at the discretion of the product reviewer.
- Cross-over is identified and applied by the product reviewer, but you are able to help by clearly identifying product grouping in the dedicated steering document provided with the online filing. This often leads to cross-over in products.
- Additional languages for labelling where required/wanted can be provided, but this is normally priced as a separate process. This is because is because translation is implemented after the regulatory review, once the Pack Copy for the product artwork has been confirmed in English.
Prices provided at quotation stage are based on the product being a new-development which will require more attention. Existing products on the market will normally have clearly established support files requiring less review time and so the costs will reduce accordingly. The online filing has a structured layout to assist you with ensuring you have the relevant supporting data on file.
Further guidance is provided on this when you start working with us.
The monthly Authorised Representative charge is a fixed cost to keep the process simple. Unlike competitors, we do not charge per annum (no-one likes a large bill and we do not trap clients in agreements). Instead, the A.R. Service charge is billed monthly in arrears with the requirement that one month’s notice be given if you are stopping the service on the product.
By charging the review price separately allows us to offer you the following flexible options:
- Defer starting the A.R. Service – so you can have the product reviewed ready to go and then start the A.R. Service once you are ready to ship. We encourage you to have products reviewed as early as possible to avoid bottlenecks with large volumes of product lines or situations where you have gaps in supporting documentation.
- If your product is seasonal, you only have to pay for the time the product is for sale (‘on the market’). When the product is no longer being sold, you can stop the A.R. Service and initiate it again at the start of the next selling season. We only require one month’s notice when stopping or starting the service. Any alterations or updates to the product and PIF need to be communicated with the product assessor in the CERT team.
- Products can be represented for as long as required with the shortest window being 1 calendar month. There are very rare instances where a product will be on the market for less than a month. If you do and you have large volumes of product lines, please get in touch to discuss your requirements.
The only key requirements are:
The product needs to have been reviewed and written approval provided of the suitability for the intended markets you want to sell in.
The A.R. Service has to be initiated prior to the product being shipped.
The discounts are accumulative and based on quantity of product lines/SKUs. The more product lines you have, the average price per SKU reduces accordingly. The discounting is ‘front-loaded’ to help keep costs down. This is particularly helpful if you are just breaking into the export market:
| Product 1 | 10% discount |
| Product 2 | 5% discount |
| Product 3 | 3% discount |
| Product 4 | 2% discount |
| Product 5 and subsequent products | 1% discount |
The 1% discount applies for the first 40 product lines after which a fixed cost per product line is provided.
If your product range exceeds 40 SKUs, we will be able to confirm the price per additional product SKU.
Yes. In the EU market, the formal term is European Authorised Representative (EAR) and in the United Kingdom as UK Authorised Representative (UK AR). The service can be referred to in different ways, including:
- A.R. – abbreviation for the generic term Authorised Representation used in both the EU and UK markets
- E.C. REP. – abbreviation for European Community Representative and is used for medical devices as an example
- U.K. REP. – abbreviation for the United Kingdom Representative and is used for medical devices as an example
- F.B.O. – abbreviation for Food Business Operator used in the food industry
- R.P. – abbreviation for Responsible Person used in cosmetics, pet food and general products (applies to both EU and UK market)
- U.K. A.R. – abbreviation for UK Authorised Representative
- E.U. A.R. – abbreviation for EU Authorised Representative
- E.A.R. – abbreviation for EU Authorised Representative
Regardless of the product category or how such a legal body is called, the main function of an AR is to ensure compliance with local market requirements and to act as the legal representative for your product.
The key legislative market obligations you need to meet are:
- Authorised Representation of your product in the markets you are exporting to. This provides assurance to local market authorities there is a local entity that is legally liable for the product being on sale in the market.
- An Importer of Record (IOR) who will handle all your customs and excise requirements for bringing product into the market.
- For the EU market, a fiscal representative (particularly if you are selling business-to-consumer) who can address your VAT requirements.
- You also have your Extended Producer Responsibilities (EPR), which are product and packaging waste registration requirements to meet Waste Packaging Directives. In the EU this normally requires an EU entity and as it is a fiscal related, it is best handled by your Importer (Importer of Record or Fiscal Representative).
When CERT acts as your Authorised Representative, we take on legal liability for your product for your chosen market and are held accountable, providing the point-of-contact for the local market authorities. The Service Agreement we agree, confirms that CERT will take responsibility for any legislative requirements we have requested or approved. For example, if we have omitted to ensure mandatory recycle information for the Italian market, or you are missing the Triman logo for France; we will be accountable for this omission.
If the product has failed due to incorrect testing by a 3rd party or faulty manufacturing process we are happy to provide support, but the responsibility will rest with you as the product owner to resolve. As the Authorised Representative we will assist with communications with relevant market authorities as and when required.
We have a thorough review process and never had an issue to date. Our job is to mitigate your product risks and we like to keep it that way!
The Product Information File (PIF) is a legal requirement for CERT who is acting as the legal representative for your product to hold a copy. This is to ensure we can provide market authorities with the information they require on request.
Some clients make copies of their existing product files, which is fine provided any changes to products are updated in the filing and your nominated product reviewer is kept updated on changes. This is to ensure the product remains compliant for the intended markets. We also have clients that use the secure filing as their main technical reference which is also fine. Well, it does save on duplication and additional administration…
There are other reasons for the filing – for example for insurance purposes, but we also use the information that you upload to complete the check. We like ‘win-win’ scenarios…
PIF details:
As an overview the following details are recorded per product:
| Product Artwork | This will be your current or proposed artwork in preparation for review and comments. The artwork can then be replaced with any updates required for the artwork, for example the Authorised Representation address. |
| Product Declarations | If you have any formal declarations required by legislation or declarations to provide due diligence, these should be saved in this folder. |
| Product Manual or Construction Details | If you have a product manual or any technical drawings, measurements and tolerances should all be recorded here. |
| Product Registrations | Proof of any formal legislative registrations or registrations to 3rd party establishments e.g. FSC should be saved here. |
| Product Testing | Health and Safety testing is mandatory to have recorded here and any performance testing which will help substantiate the product is recommended. |
Any information that a marketing authority may ask for to substantiate your product claims or to confirm suitability for the market and intended use.
Each product line (SKU) will have its own PIF with a standard filing layout as shown above. This is for quick and easy reference to the details if needed and to comply with legislative requirements. More detail is given when your online filing is setup.
You need to remember that if your product is challenged by a marketing authority, we need to be able to readily share relevant details to substantiate your product. You wouldn’t want your entire product range shared and we don’t want to irritate authorities by dumping excesive information on them, or delaying their request while we sift through the data to supply.
The purpose of the PIF is to have the relevant information quickly available to demonstrate compliance if challenged. We don’t want to draw unnecessary attention to other products which we refer to as “Snow Balling”…
Situations where you can have the same products share a PIF:
- Variarions in size e.g. garments or a food portion size – where there is no change in materials, technical requirements or regulatory requirements.
- Variations in colour or finish where the change does not affect testing or regulatory requirements (a minor addtion such as an extra test report which is clearly identified in the filing is acceptable).
If you have questions in this area, we are happy to navigate you through this when we review your products with you.
All information you provide is held on a secure server (Office 365) using TLS1.2, encrypted data traffic using TLS and IPSec and multi-factor authentication.
You are provided with two-levels of access:
Read/write access – allowing you to upload and amend documentation on file and download any documentation you need. This does not allow bulk deletion or filing restructuring to help keep your documentation secure.
Administrator access – As this is your data and you own it, a nominated user(s) – the preference is to have a single administrator for simple clean point-to-point communication – who has a more secure access to the folder and is able to amend the details however they wish in the filing.
CERT take pride in providing clients with a quick turnaround on projects and officially aim to approve products within 5 working days. Realistically it often is sooner than that and depends on current work volumes. We appreciate your projects are important to your business and this requires good project management from us to avoid ‘bottlenecks’. You can help by submitting products as soon as possible for review, particularly for large volumes of product lines/SKUs. This helps to build in suitable time margins for us to ensure the products are correct and allow you time to deal with any gaps in information needed to complete the review.
The team cannot emphasise how important it is not to leave assessments to the ‘last-minute’, as projects under time pressure (i.e., needing less than a 24-hour turnaround) will incur a 25% surcharge on the assessment and does not leave any time margin to handle any missing information.
Once the Service Agreement has been approved, CERT will have your online secure filing up and running within 24 hours. So, then it will be how quickly you can populate the information into the PIF. Realistically, if you have an existing product you are looking for Authorised Representation for – provided you have all the details to hand and updated artwork, you could be ready to “push the button” within a week!
As already mentioned, your information is secure and is only accessed by yourselves and your CERT nominated regulatory professional. You may have instances where a 3rd party involved in your product distribution may ask for confirmation. DO NOT give them any secure links you have been provided with for your data, instead we recommend one of the following:
- You can download and email a copy of the declaration provided by your Product Reviewer, confirming the product suitabililty which states the markets it has been confirmed for.
- You could download the product folder and email it, but this may be bulky and puts your intellectual property at risk.
- For a small administration charge we can setup a temporary, read-only secure link, restricted to the person who requested it. Please note they can download information while it is available to them, but cannot alter or add any details to your product information. This is the secure option for sharing a folder of information.
- The most secure option – is access to a single document in your folder, that can be shared with a designated person, within a specific timeframe. The document is read-only and cannot be downloaded.
If you need one of these service options, please contact your dedicated Product Reviewer for support.

