The Do’s and Don’ts of the CPC Process
The CPC Document has been engineered to be able to provide suitable Pack Copy for artwork generation all within the CPC document. This is great, but it is supposed to be the exception to normal procedure.
Normal Procedure:
You are requested to complete the Sourcing Information on the CPC Document.
- Once the details have been completed, the example information can be removed to save space by clicking on the action button on the Front Page.
- The document can be emailed to the relevant person in the Product Development Team.
- The Product Development Team will confirm if the product is to progress, upload it to the database and invite you to complete the specification in the online database.
Option 2:
The CPC Document can be bypassed altogether by the Product Development Team. In this situation:
- The Product Development Team will setup the product structure in the online database and you will be invited to complete the Sourcing Information on the database.
- The Sourcing Information is submitted for approval via the online database.
- The Product Development Team will confirm the product is going to progress and you can then complete the specification in the online database.
Option 3: (with permission from the Product Development Team)
- The Sourcing Information is completed on the CPC Document and sent to the Product Development Team for approval.
- Once approved, the specification is completed on the CPC Document and submitted to the Product Development Team.
- If the specification is correct to progress, the Product Development Team will create Artwork Pack Copy from the CPC Document and upload the document to the database at an appropriate point.
- You may be requested for further details needed for the database, or depending on the reason for using option 2, you may be invited to the database to add in any missing details.
DO NOT:
X – Progress from the database back to the CPC Document. The database does not communicate back to the CPC Document (it is a one-way system) and any information not on the CPC Document will be lost.
When you complete the document, you will see the progress of your completion shown on the Front Page as a percentage next to the section. This normally has to be complete to progress to the next section, however if the Product Development Team do not require this, it can progress due to the flexibility of the process. The Critical Path summary section on the Front Page will provide different values, depending on whether you are completing the Initial Submission format, or the Complete Document format. You can quickly determine by looking at the Front Page, which document format you are using:
Initial Submission format:

Complete Document format:
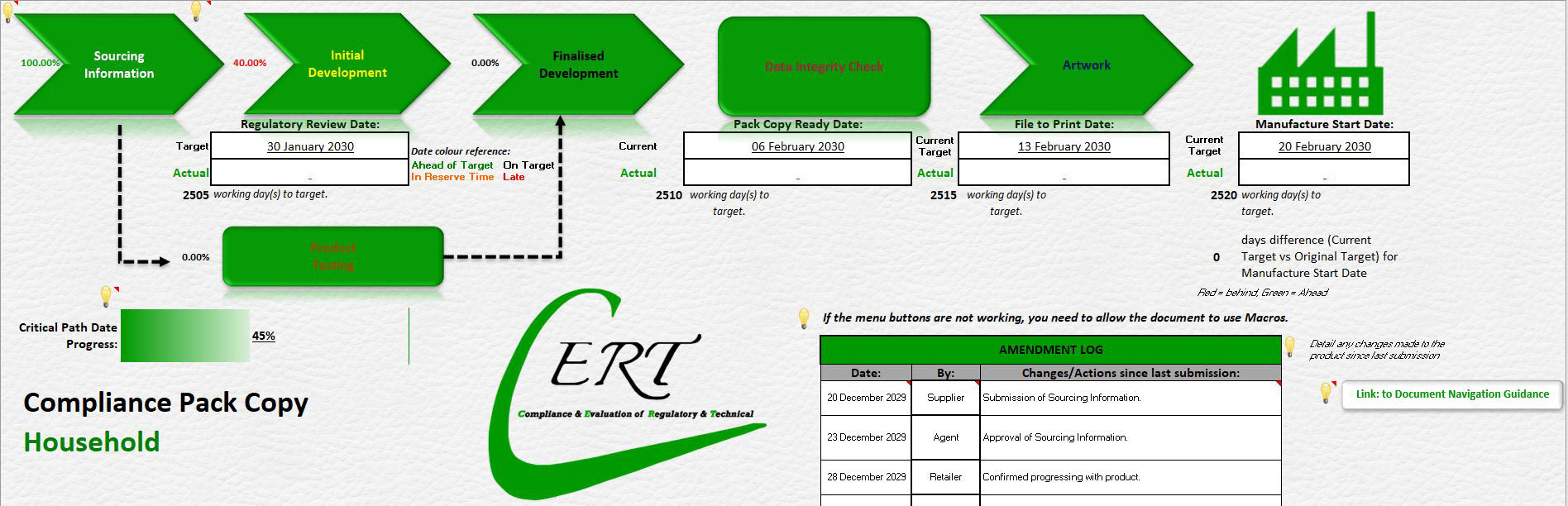
The Initial Submission format refers to the summary as “Critical Path Setup Progress”, so is only checking the information required to setup the critical path.
The Complete Document format refers to the summary as “Critical Path Date Progress” and so takes into consideration the actual dates completed for the process. So this will only show 45% complete when the critical path has been setup. This is normal and the progress will only show 100% at the end of the product development. The CPC Document should have been uploaded to the database long before that.
The process is engineered to add and hide sections based on the relevance to your product. There should be no need to delete sections in the process.
If you add a new line in the document and it is not needed, just remove your input details from the line and it will not update to the database. Alternatively, if you contact the Product Development Team, they should be able to arrange for the line(s) to be removed if needed.
If you are concerned it will affect the Artwork Pack Copy, click on the Artwork section which provides a live update on the Artwork Pack Copy so far.
In the CPC document you are able to expand the rows for more space to fit the text. If you cannot fit the text within the allowable expansion, will you really be able to fit the information on the product label?
If it is green coded (i.e. technical supportive information), the data fields are there to provide a quick summary. In-depth explanations should really be provided as separate attached documents in the ‘Product Supporting Documentation’ in the Product Testing area. If you are attaching supporting information linked to a data field, it is recommended to make a comment that supporting documentation has been attached so that it is clear.
The supporting videos on the previous page also show examples of how you can add rows to the section of the document that can have multiple entries. If you need extra rows and there is no option to add new rows, are you placing the information in the correct section? It is recommended to get in contact with the Product Development Team/Technologist for advice.
If the answer is not on the Guidance Page, then below is a quick overview of where core data should be placed:
- If your information is Product Testing, Product Declarations or any Supporting Documentation, then this needs to be placed in the separate ‘Product Testing’ section accessible on the front page. Supporting Documentation can be anything from HACCP documentation, drawings, size charts, Safety Data Sheets plus more….
- Section 2 is what we like to call the Breakout Section. This area captures product areas that will have dedicated regulatory requirements e.g. CLP, Pet Food, Dosage Tables…. The lower half of Section 2 is where the majority of the product labelling information is placed. ‘Product Logos and Pictograms’ addresses visual instructions, followed by any text instructions and then product registration marks e.g. trademarks, copyright and official registration marks.
- Section 3 has the facility to communicate any markings on the product itself, meaning it does not need to be present on the packaging, followed with the standard usage, care and warning labelling (that does not have a dedicated area e.g. CLP) for the product.
- Section 4 of the specification has an area to list component details, which can be anything from 1 to however many you can fit into your product!
The Specification is laid out in 11 sections:
- Identification details.
- Product specific labelling requirements.
- Storage and usage instructions.
- Product composition details e.g. components/materials used.
- Allergen information.
- Quality attributes.
- Product/factory processes.
- Product safety and shelf life.
- Packaging.
- Declaration confirmation and safety website details.
- Critical path / actual development dates.
If you still have a question, please start your enquiry with the Product Development Team who will be able to direct your query to the relevant team.
The CPC process has been designed in English, as this is the mother tongue of CERT, however we work with multiple translation houses. It is also important that you communicate in a language your Development Team understand well to ensure nothing gets missed. By default, the CPC process is provided in English. If you need it in another langauge, please discuss with your client/development team who provided the document to you. Alternative languages are available for the document, but currently the online database is only available in English. CERT are constantly making improvements with a multi-lingual online database being an option in future.
There is no need to print the main page as this is just supposed to be a dynamic summary of the data in the document. The Initial, Final Specification and Artwork Pack Copy are all printable if you need a hard copy.
If you are printing it, why not save it as a PDF and avoid using paper? Depending on your version of Microsoft Office when you go to the print option, instead of selecting your printer, there is normally an option to print to PDF.
CERT spent a long time getting the formula right in terms of what information to place where and so don’t want the information readily copied and inferior versions created. CERT take pride in making efficient systems that work and thank you for preserving it for this reason.
This is not readily changeable to ensure consistency, however not everything is perfect. If a field name needs to be changed, contact the Product Development Team who should be able to arrange the field change if your query is feasible. Field Names can only be changed through the relevant Product Development Team.
As shown in the video on the previous page, if you need to add an image there should be a button called “Manually add an image”. If you click on this button and follow the instructions you should be able to add an image. Don’t forget to click any ‘Apply’ button afterwards to save the image and settings you have just added.
If you have locked a section e.g. Section 7 of the Sourcing information and need to change a detail, please contact your Product Development Team who should be able to help.
Often you may have a range of similar products and it can be laborious retyping the same information. The CPC Process is about working smart. As the process starts with the CPC document, all you need to do is take a copy of the document, rename it and change the appropriate details.
WARNING! From experience this is an area where mistakes occur, as the emphasis is on the user to make sure they capture all the data changes needed. Always double check you have activated and deactivated all the appropriate sections as well as changed the relevant data.
Section 2 of the Sourcing Information, has guidance on when you can put all the variations on the same specification. If the product variation has changes in regulatory or technical requirements, then they will be classed as a separate product and will need their own specification. An example is where the volume of a chemical product has a different Classification Labelling and Packaging (CLP) warning. This is a change in regulatory labelling and so would need a separate specification.
Handy Tips!
Etiquette on completing data fields.
When filling in the detail. It is very easy to type the data in the top navigation bar and then move onto the next field. This can result in the row not being adjusted to fit the text placed in. The ‘Wrap Text’ option is activated on the relevant data input cells, but is dependent on how you input your data. Wrap Text does not always capture spacing needed if you copy and paste data for example.
So by adding a full stop at the end of your data input, confirms that it is the end of your statement. It is also handy for yourself when adjusting the row, so you know you have not cropped off any information. The cropping does not affect the database as this will take all information (shown & hidden), but I am referring more to the Pack Copy/Artwork preview, or reviewing data on the CPC Document.
If the full stop must not be there i.e. it is a blue section that pulls directly to the Artwork Pack Copy, then don’t add one, but make sure the field is not cropped. Talk with your Product Development Team as this is generic guidance, they may have a particular preference.
Some data fields have more than 1 row merged together. This is because we anticipate you may need more space, than 1 row will allow for data.
If you click in the very left hand column (the row numbers), it highlights the whole row. Click on the top row of the area you want and with the mouse button depressed, move the mouse down to highlight all relevant rows. With the rows highlighted, move the mouse between two of the relevant numbers so you get the ‘double arrow’ spacing symbol. left-click and drag to the size you need. All the highlighted cells will adjust evenly to the same size as selected.
Placing a dash “-” in the field makes it clear to the readers further in the development that you have reviewed the data field and it is not required in this instance.
To add a dash in excel, click in the cell, press the space bar and then add the dash. If you do not place the space before the dash mark, excel assumes you are trying to perform a calculation!
The space, then the dash indicates to Excel it is a text field.

