
Welcome to the Food Business Operator Service which comes under the official term Authorised Representation. We assume you have received your link to securely access your filing. Please don’t forget you have two levels of access to the filing:
(level 2)Administrator Access – which is linked to your email if you have been invited to this. This allows you to move folders, do bulk uploading and deletion and gives you full control over the filing.
(level 1)User Access – Which still allows users to upload and download documents, make edits and simple document movements in the filing. This is through the secure link and password provided and is accessible to anyone given this link.
The video above is a quick visual guide to how our filing system works and is here to compliment the written guidance that is also available on your dashboard – “a picture paints a thousand words…”
 | All of our demonstration videos can have the playback speed adjusted, as we appreciate everyone reviews information at different paces. You can also use the slider bar at the bottom to navigate to different sections in the video. If the image quality is low, YouTube has opened on a low resolution which can be changed to a recommended mimimum 720p, but 1080p (HD) is best. |
Key sections in the video:
- 0:00:20 –> Layout and options available on the online dashboard
- 0:02:44 –> Using your Product Schedule
- 0:03:37 –> Adding a product to your Product Schedule
- 0:06:28 –> Updating us of the Stage 2 starting date
- 0:07:50 –> Using the product filing (how to add data)
- 0:08:55 –> Admin Access – Creating a new Product File
- 0:10:49 –> Copying information from other product files
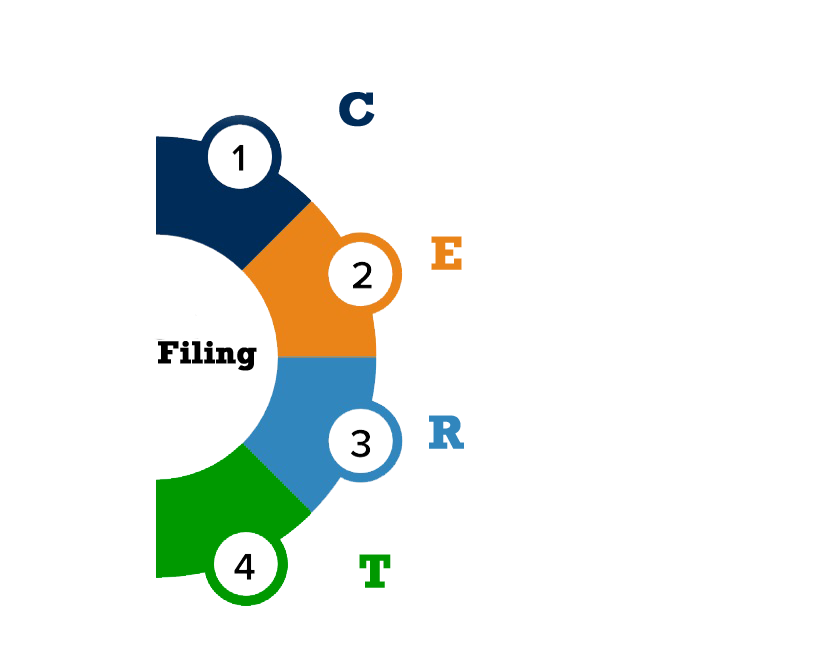
Breakdown of the filing requirements:
- A low-resolution image (ideally PDF) of the final product artwork that is being used on the product on point-of-sale packaging.
- A copy of any instructions provided inside the point-of-sale packaging needs to be saved in this folder as well, to ensure a visual representation of all information supplied with the product (excluding shipping information) is present.
- This is any official certification, approval certificates from 3rd party endorsements. An example could be vegan society logo.
- A company statement declaring that the product meets food safety standards or relevant documentation i.e. HACCP system in place and or GFSI certified manufacturing.
- Finished product specification details such as recipe, allergens, weights, nutrition, micro limits, packaging, shelf life.
- Product specific supporting documentation/ statements from manufacturers i.e. GMO statements, allergen risk assessments.
- Any testing specified in the regulations and directives relating to your product i.e. gluten free claims, sulphite testing.
- Analytical testing i.e., nutrition, microbiological testing.
Your online dashboard is the central hub for all your support and filing activities which links to your Product Schedule. The Product Shedule is the ‘Go To’ document to see at a glance the status of your products, but also to guide us as to which products to review and what markets to assess and approve a product for compliance with. For this reason, it is vital it is kept up to date and is the central driver for your projects.
You can request product reviews, specify targets and review priority order of your products from the Product Schedule. By specifying the immediate markets you require, you can save time and cost on the initial review. You are always welcome to add additional markets at a later stage for confirmation of product compliance. We actively encourage this as it means your export business is growing!
Don’t forget!
- As shown in the video, there is written guidance (PDF and Note) on how to successfully use the filing.
- It is important to ensure you complete the Product Schedule. This is our instruction to make sure we have reviewed your product for the correct markets and helps point out potential cross-over to save review time.
- Your product can only be shipped into your target markets once you have obtained written approval on file from your nominated regulatory professional member in the team. This will help ensure your product is compliant with the market requirements and avoid it being detained or investigated.
Please visit the FAQ Page for further advice and support regarding the filing. If you still have questions, the team are happy to provide you with support. Please feel free to contact the person responsible for your product reviews.
The filing can only be started once you have been granted access via Administrator or User Access to the folder.
Once you have completed the product information as per the video guidance and PDF guidance provided in the filing, then you need to follow the simple steps below:
1. Create a Product File as per instructions and demonstrated in the video on this page.
2. The file will not be touched until you invite your dedicated regulatory specialist to review your product.
3. When your Product File is complete and ready to be reviewed, ensure you have completed all the details on the Product Schedule. The Product Status column will confirm this and there is further support in the Product Schedule if needed.
4. Do not make any amendments while the product is under review. IF you need to make a change – Then please contact your nominated regulatory specialist and confirm they are happy for you to update the file. This ensures data does not get missed in the review!
5. If changes are needed to your data submitted, this is normally communicated directly with you in the form of a checklist, but at the discretion of the approver can take the form of an email or a call if this more appropriate.
If your Product File is in order, it will be approved and a PDF certificate of approval will be emailed to you and a copy saved in the Product File.
6. The product is now released to be distributed to your nominated markets. If you have not specified a date you are looking to ship into the market, the service will start with immediate effect. If you have requested to delay starting the representation service, the service will start on your nominated date. If you want to delay the service starting, please communicate this to your dedicated regulatory adviser as soon as possible so our systems can be updated accordingly.
Please refer to the FAQ page and PDF guidance in the filing for additional support on the filing process.
This is a password protected section. If you have not been provided with connection details for this area yet, please be patient they are probably en route. If it has been over 48 hours since we had the pleasure of you joining us, please email [email protected] to request access.
The secret ingredient is keeping the process simple with clear concise information. We appreciate you have targets and deadlines and we try to help as much as we can, but we are not magicians. As per the tips on the FAQ page, the more time you can build in for product review and the more complete your filing, the faster we can help get your product to market!


